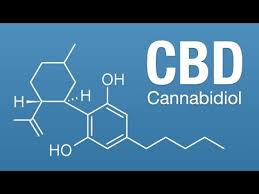
About SOPs for CBD and Hemp Derivative Producers
Standard Operating Procedures are the backbone of a certifiable document management program for Food or Dietary Supplements manufacturers.
The entrepreneurial smaller manufacturing companies In the hemp derivative sector are at a competitive disadvantage without a cGMP document system. The real issue here is the actual expense and investment required to hire a 3rd party regulatory & […]