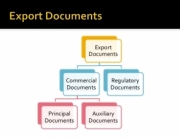
Batch Record means Manufacturing documentation, packaging documentation, and exception documentation, such as a non-conformance report, Deviation Report and Certificate of Analysis, and additional documentation, which may have been processed as part of the production and packaging records of the Batch; as defined in 21 CFR part 110 and/or 111, or other certifying bodies as specified in codes for compliance. It is an intrinsic part of assuring that the end product (finished Good) is manufactured consistently to meet the conditions for the end product as specified in the Product Specification.
Most of the time I find that companies when undergoing an outside audit in this space are using an inadequate and non-conforming MMR (Master Manufacturing Record in the PPC (Production Process Control Chain). Additionally the Quality Control aspect of the PPC is lacking written internal controls to ensure the safety of the product. If these controls (SOP’s) are non-existent or not applied to every batch, then consistently meeting the Product Specification is impossible.

regulatory compliance concept on the gearwheels, 3D rendering; Shutterstock ID 642096322; Brand: NO; LocationProduct: Mag; Date: 8/17; Purpose: Edit
I specialize in the creation of compliant quality cGMP Document Management Systems at an affordable price for smaller manufacturers and processors who think they cannot afford it.
If this is you, just call or email me. I am committed to helping you bring your concern into compliance.