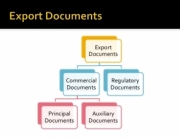
The smaller and innovative companies often do not have the personnel or knowledge bank to handle and successfully provide the ever-increasing document load to gain import approvals..It can be very time-consuming and often the “paper trail” is so multi-faceted that it seems to be “not worthwhile”. Health Ministry approvals can take a long time, sometimes as much as 6 months.

The first gate that a supplement or functional food brand and manufacturer must go through is having a recognized Food Safety Certification which means that the manufacturer has invested the time, money, and resources to achieve a globally recognized certification. This can be a cGMP certificate issued by a 3rd party private organization. Then those certificates will need to be authenticated by governmental authorities, and in some cases, also by the foreign consulate or embassy. In the case of dairy containing products, often a health certificate from the USDA is necessary which will also require additional documentations pertaining to the raw materials used in the production of your product.
A manufacturing Flow Chart can be required.
The list goes on depending on where in the world the importer is located. As one can see, this can be a convoluted effort.
Nutrition 50 has been helping companies for over 25 years in the international export markets for U.S. based companies in the dietary and Functional Food consumer product space. We specialize in the Technical and Regulatory arena.
We can help you get your products through these mazes. Call or email us to have a conversation.
We create possibilities……