Are you a small to medium-sized branded dietary supplement marketer? Do you have a warehouse where you store and ship nutritional supplements or functional foods to your customers?
Do You have contract manufacturers that provide you with products? Do you have cGMP standard operating procedures written and in place for your operation? Are they kept up to date? Did you know that the FDA inspectors can show up unannounced and request an inspection of your facility?

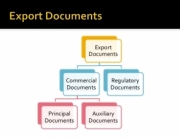
There is a clear path to compliance and risk reduction for dietary supplement distributors, marketers, and brands. As Holding Facilities for nutritional supplements, you must ensure adherence to cGMPs under Subpart M of the CFR 21 part 111 or 110 cGMP rules. This involves having the necessary written S.O.Ps and an accompanying Document Management Control System (DMCS) in place. These systems ensure compliance and increase efficiency and safety, offering a clear advantage to your operation.
Primarily is the risk of an audit by an outside U.S. regulatory agency (i.e., FDA, USDA). The second is an inadequate HACCP (Hazard Analysis Critical Control Points) program. This program deals with identifying weaknesses in environmental control.
Form 483, also called “Inspectional Observations,” is a list of conditions or practices that indicate a potential violation of the FDA’s requirements after a regulatory inspection. The observations are listed in descending order of importance. This is not an all-inclusive list but more of a snapshot of possible issues noted at the site. FDA law is unique in that the Agency does not have to prove a product is harmful to public health, but only that there are conditions where this may occur (e.g., not following the law and its implemented regulations). The potential consequences of non-compliance can be severe, including product recalls, financial penalties, and damage to your brand’s reputation.
After an inspection, the FDA Form 483 is presented and discussed with the company’s senior management. Companies are encouraged to respond to the FDA Form 483 in writing with their corrective action plan and implement it expeditiously. This response should include a detailed plan of action to address each observation, a timeline for implementation, and any necessary follow-up actions. (Usually within 15-30 days)
When it comes to managing risk, the cost of implementing a document management and control system is not a barrier. In fact, it is a manageable investment that can bring significant benefits. By increasing efficiency and safety, these systems can significantly reduce the risk in a product recall event, providing a clear return on your investment. Investing in these systems is not just a precaution, but a strategic move that can enhance your business’s resilience and reputation.